Toxicology and Performances for regulatory compliance
The “Toxicology and Performances for regulatory compliance” laboratory (TOP) has recognized and consolidated experience in the following macro-activities that are delivered accordingly to the international sector regulations and through advanced methodologies. Furthermore, the TOP laboratory supports in study protocols elaboration and identifies the best in vitro and in vivo analysis approach to evaluate the materials activity.
The activities
Toxicology:
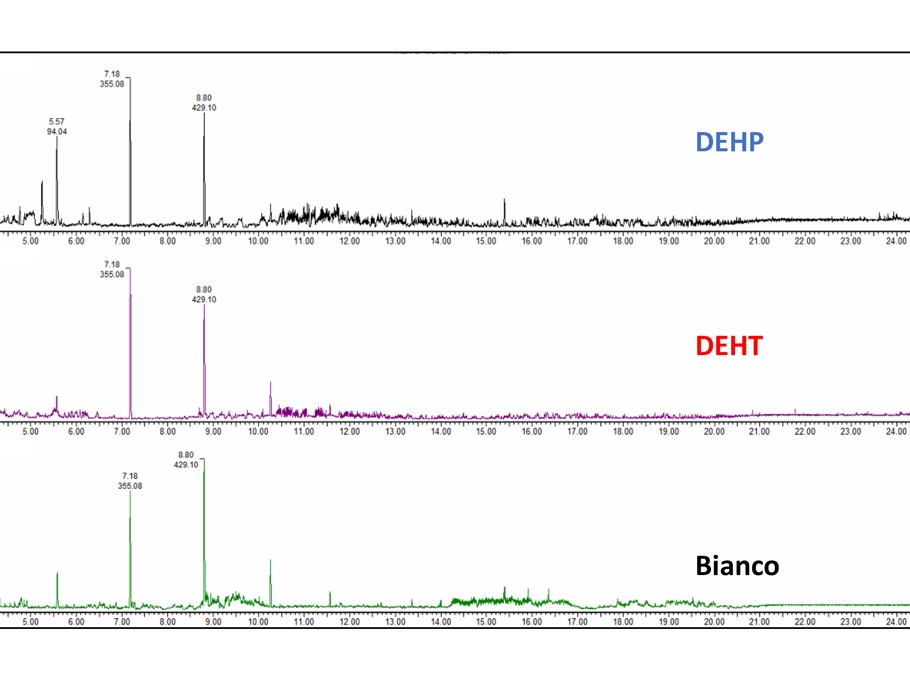
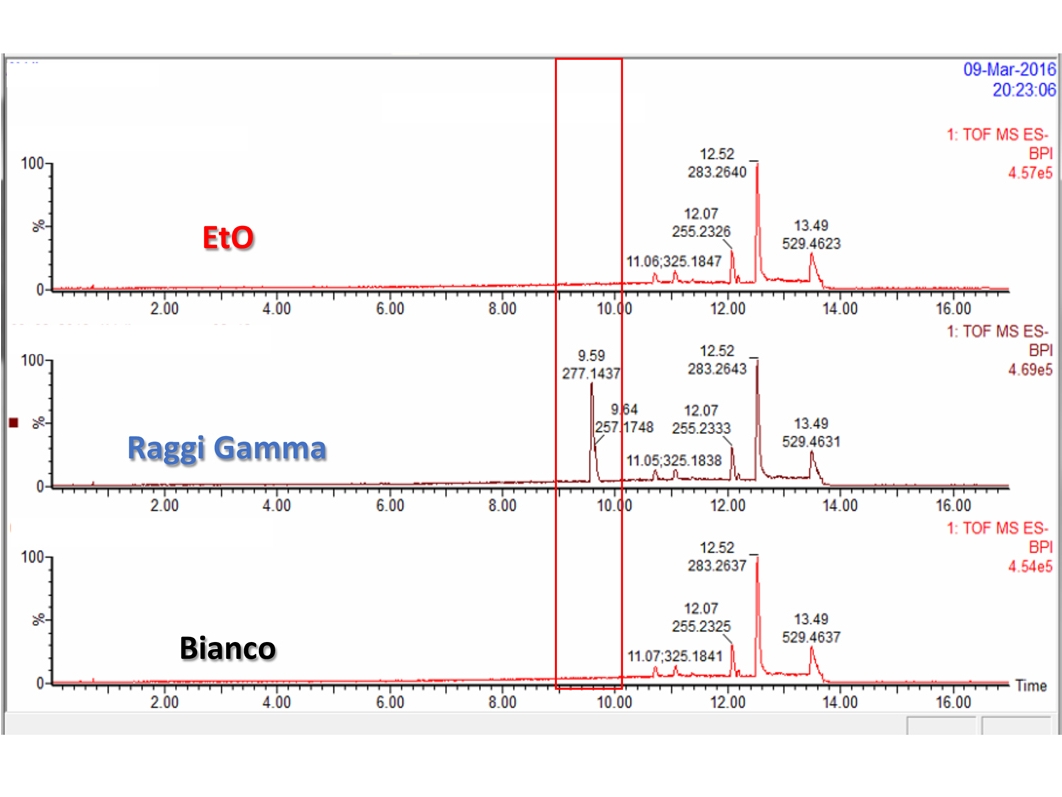
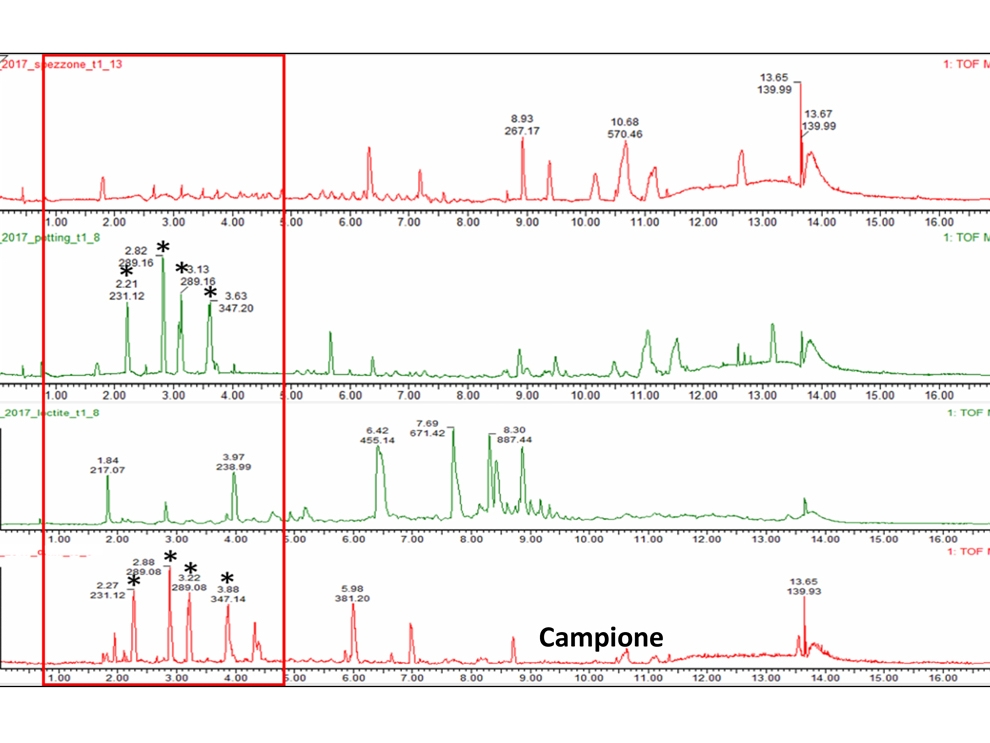
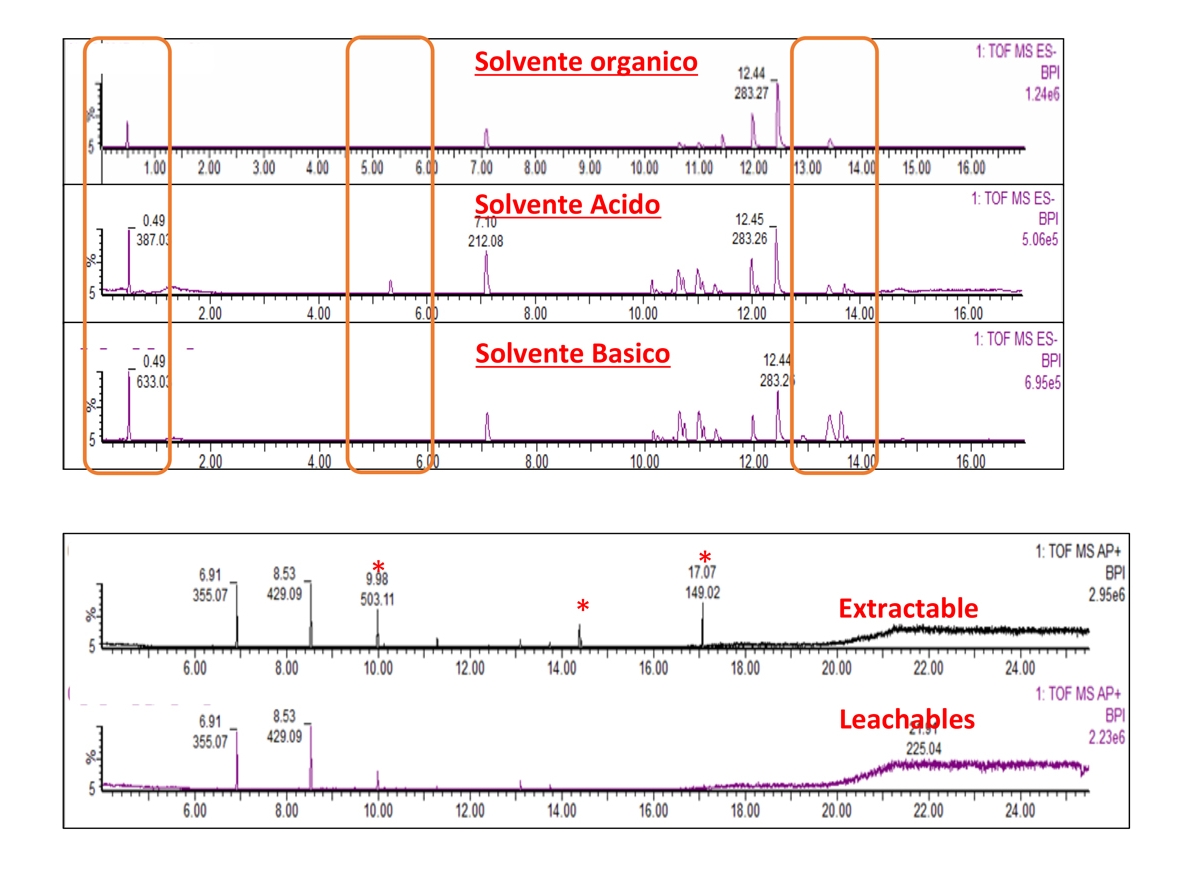
- Chemical characterization as per ISO 10993-18 (Leachables and Extractables) on medical devices in order to assess the migration of organic compounds (non-volatile, volatile and semi-volatile) and inorganic elements. These activities are performed within a quality management system :
-
- ISO 9001 (generic quality system)
- ISO 13485 (medical devices quality system)
- GLP (good laboratory practice)
- Protocols and activities related to set up and validation of analytical spectroscopic and chromatographic methods.
- Consulting on certification for CE marking, registration of medical devices in extra European countries, certificates renewal as per MDR 745 (2017) requirements and MDCG guidelines.
- Examples:
-
- Toxicological Risk Assessment (TRA) (ISO 10993-Part 17)
- Biological Evaluation Report (BER)
- Biological Evaluation Plan (BEP)
- Clinical Evaluation Report (CER)
- Clinical Evaluation Plan (CEP)
Application sectors
Dental
Pharmaceutical
Cosmetic
Agro-food
Biomedical
Scientific Supervision:
Prof. Aldo Tomasi MD
Laboratory Head
Dott. Simone Roncioni
Staff and skills
Dott. Simone Roncioni, Laurea Magistrale in Scienze Chimiche
Dott. Francesco Burini, Laurea Magistrale in Scienze Biomolecolari e Cellulari
Dott. Luca Accorsi, Laurea Magistrale in Scienze Chimiche
Dott.ssa Elena Rebecchi, Laurea Magistrale in Scienze Chimiche
Dott. Lorenzo Morelli, Laurea Magistrale in Scienze Chimiche
Marco Palella, Tecnico Superiore per l’innovazione, sviluppo e produzione di medical devices
Equipment
Toxicology
Ultra performance liquid chromatography – Electrospray ionization – High resolution mass spectrometry (UPLC-ESI-HRMS)
Ultra performance liquid chromatography – UV spectroscopy (UPLC-PDA)
Gas chromatography – Electronic ionization – Low resolution mass spectrometry (GC-EI-MSq)