Toxicology and Performances for regulatory compliance
The “Toxicology and Performances for regulatory compliance” laboratory (TOP) has recognized and consolidated experience in the following macro-activities that are delivered accordingly to the international sector regulations and through advanced methodologies. Furthermore, the TOP laboratory supports in study protocols elaboration and identifies the best in vitro and in vivo analysis approach to evaluate the materials activity.
The activities
Toxicology:
The TOP lab develops:
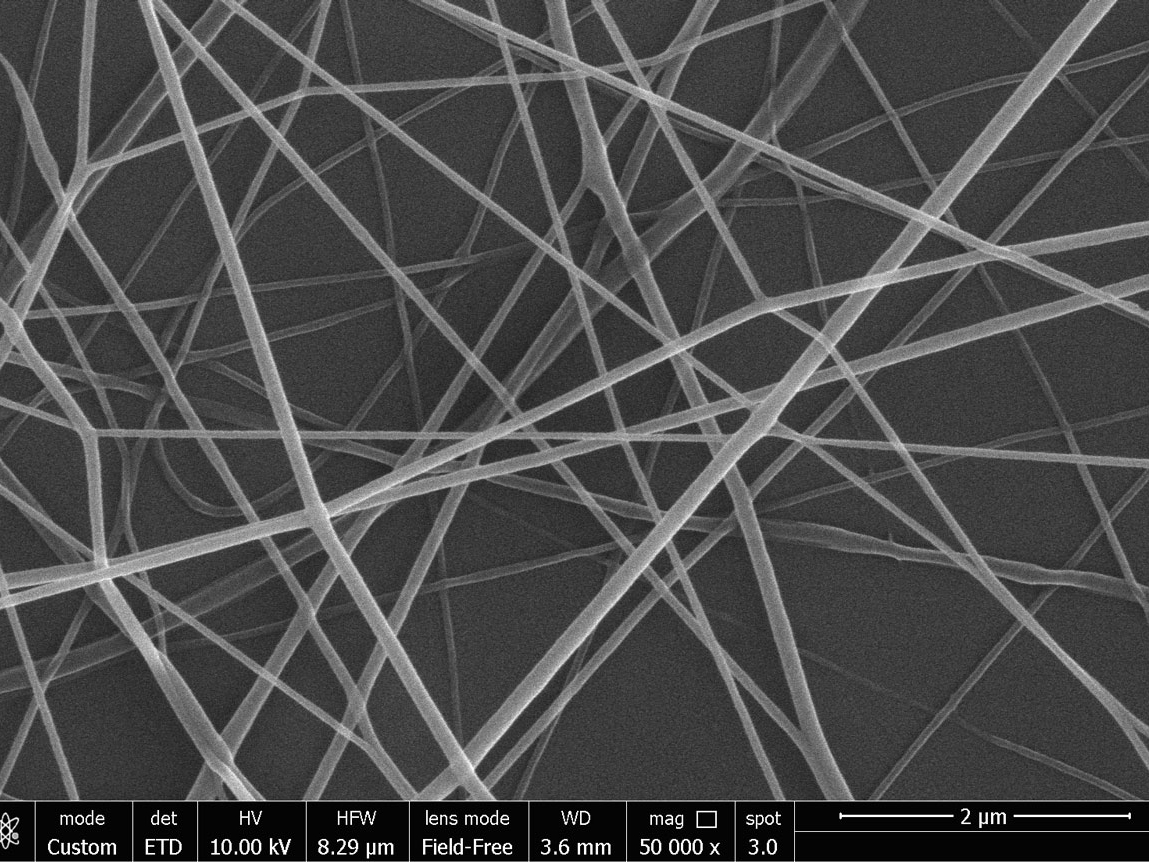
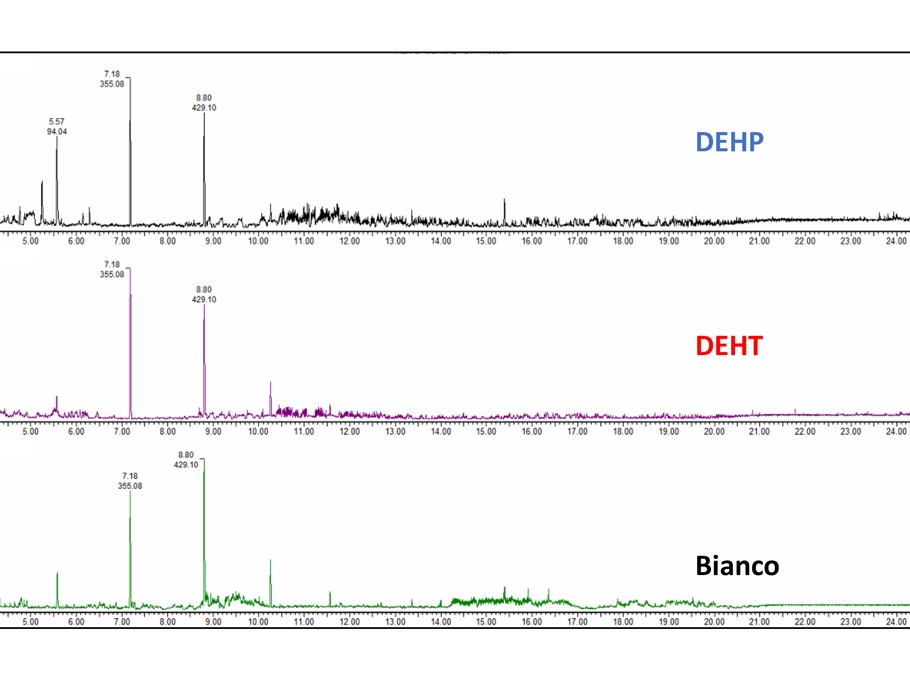
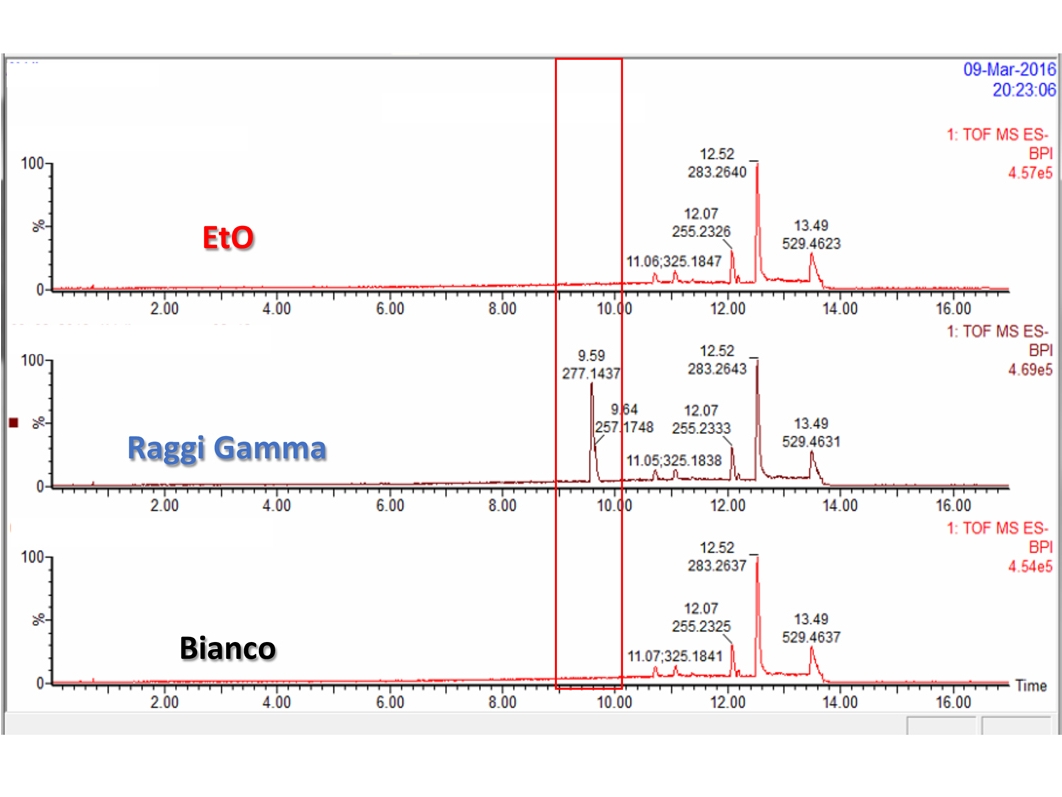
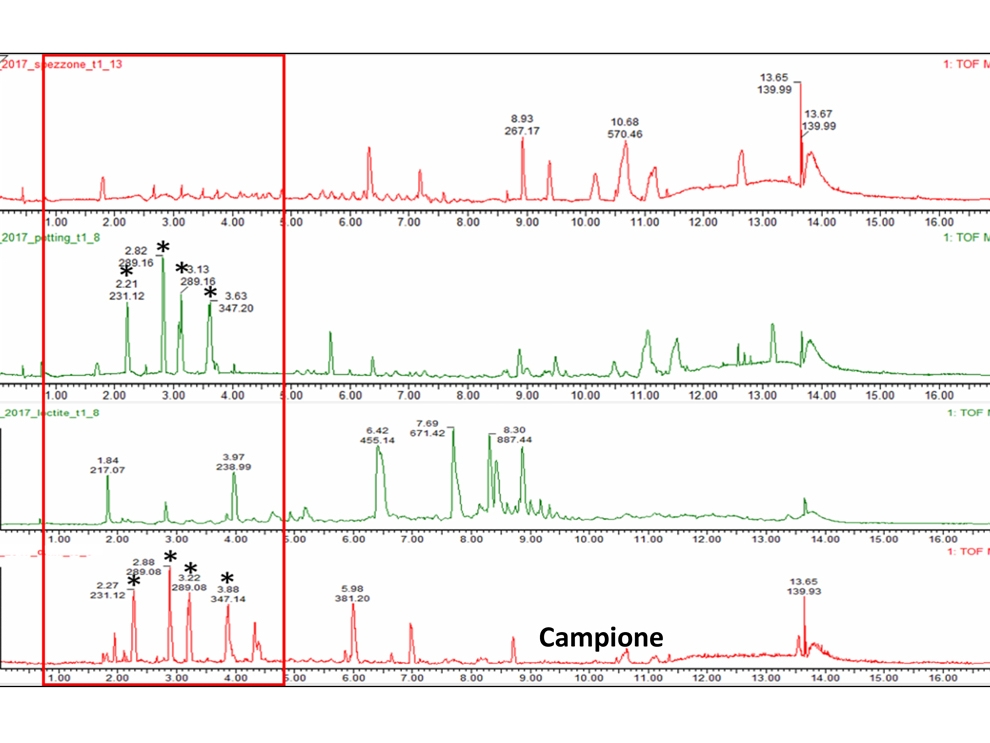
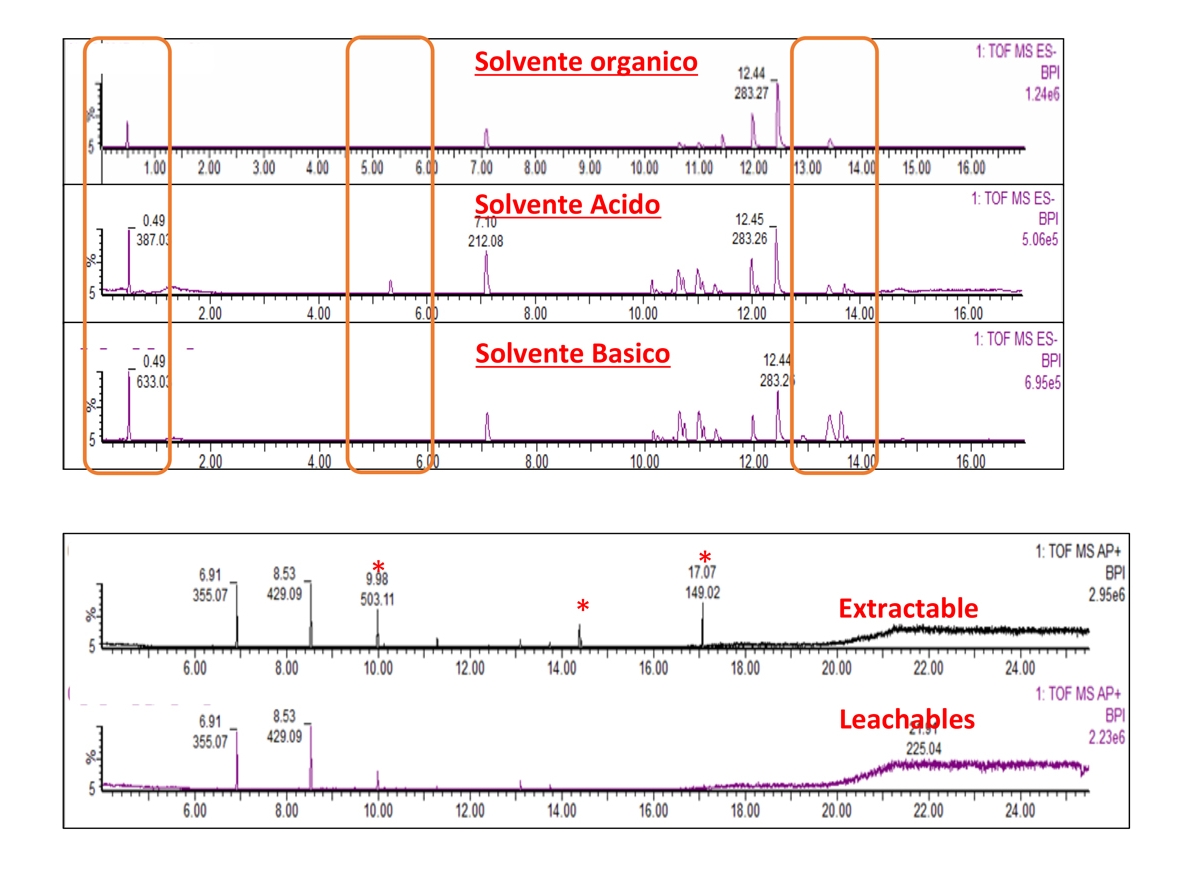
- Leachables & Extractables studies for the detection of potential contaminants in traces deriving from the packaging (ISO10993-18) under conditions of use and forced (accelerated aging) by high performance liquid chromatography (UPLC-MSMS) and / or atmospheric pressure gas chromatography (APGC-MS).
- Appropriate methods of development and validation for the determination and quantification of releasable substances or compounds of interest UPLC / APGC-QTOF.
- Qualitative and quantitative analysis of chromophoric molecules using UPLC-UV.
- Implementation of toxicology and toxicokinetics studies of substances released from materials.
- Toxicological risk assessment and Biological risk assessment according to regulations (ISO 10993-Part1 17)
- Stability testing of pharmaceutical products or medical devices subjected to changes in environmental factors over time such as temperature, humidity and light.
- Evaluation of degradation products
- Identification of impurities or production anomalies in collaboration with the chemical laboratory and the Great Instruments Center (CIGS) of the University of Modena and Reggio Emilia
Application sectors
Dental
Pharmaceutical
Cosmetic
Agro-food
Biomedical
Proteomics:
TOP lab provides:
- In vitro simulation tests for the evaluation of new membranes performance or of the new sorbent materials adsorptive capacity.
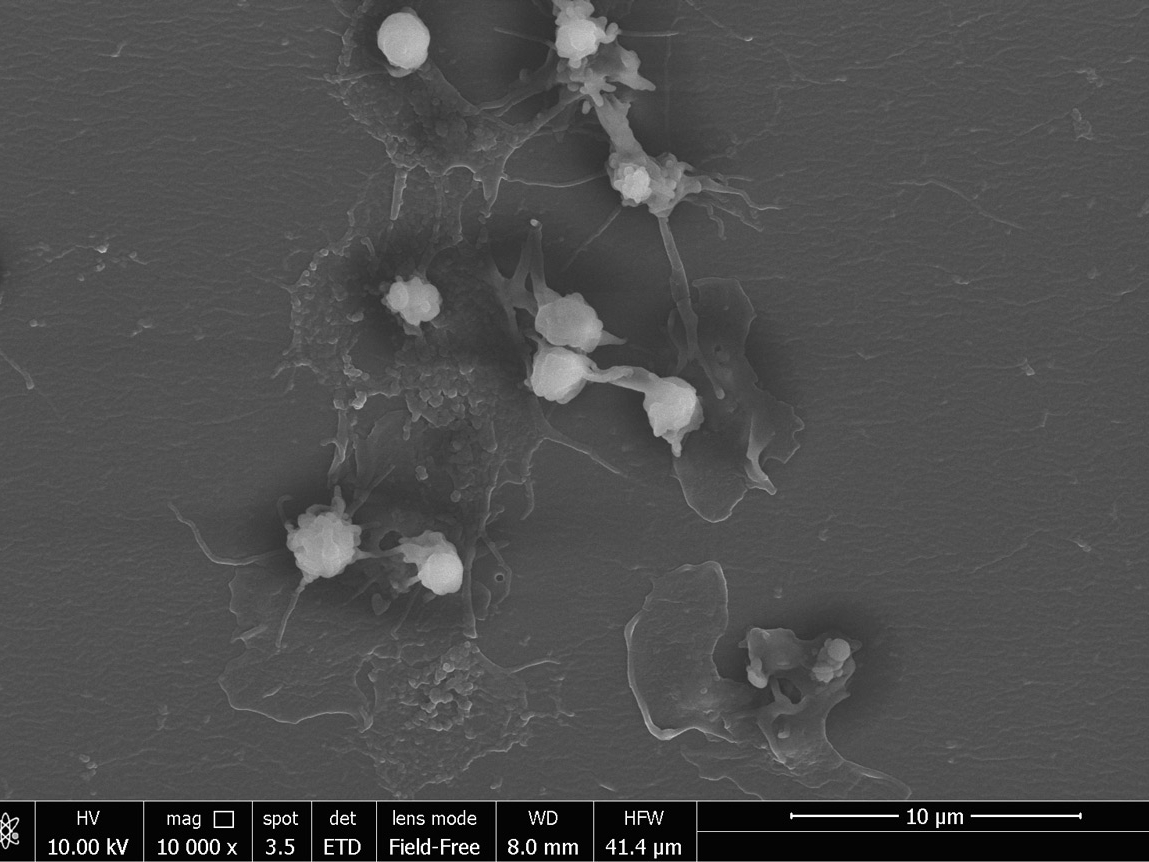
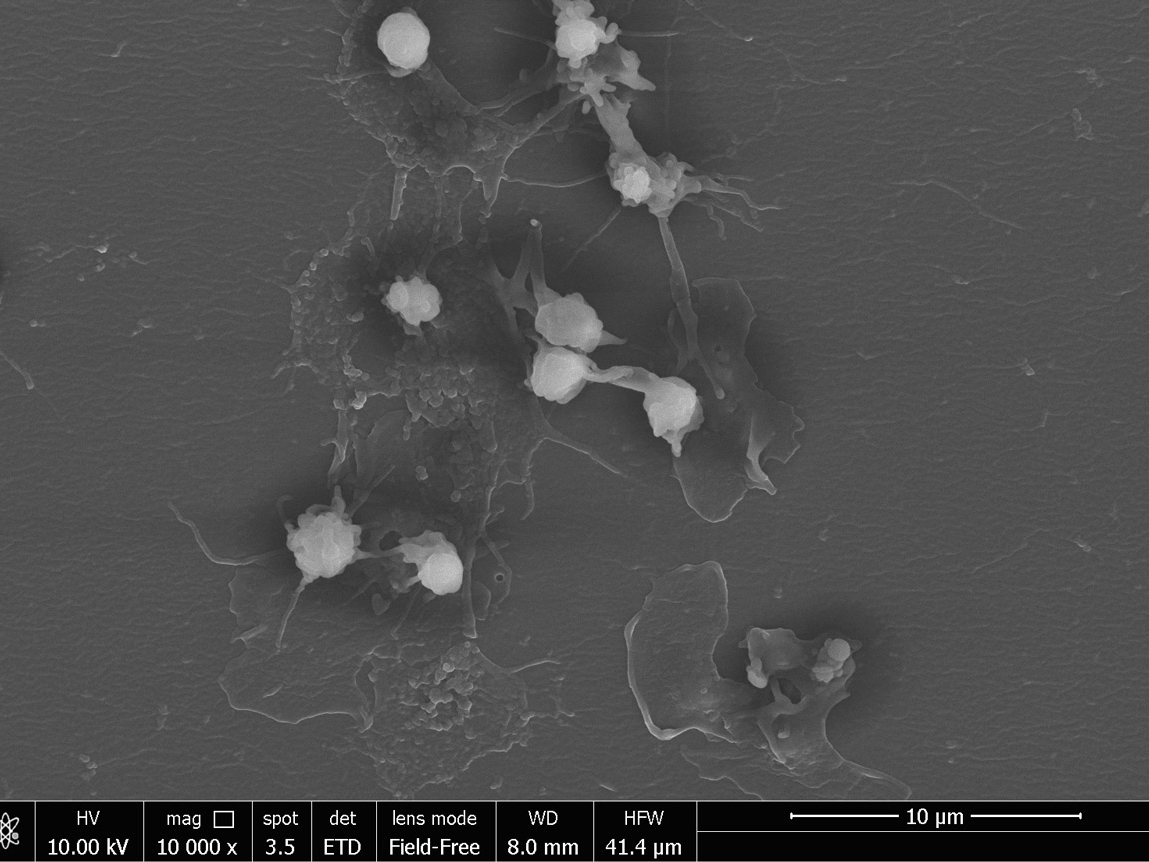
- Extraction of protein molecules from samples of different biological nature, separation of proteins by mono- and bi-dimensional electrophoresis and creation of two-dimensional maps with different sensing techniques. This type of activity was applied for the evaluation of the medical devices in vivo activity and for comparative tests for the new materials and devices performance evaluation.
- Image analysis of protein maps, protein speciesdifferentially expressed proteins matching and evaluation.
- Processing of protein bands / spots for mass spectrometric analysis (Nano HPLC-QTOF).
- Processing and querying databases for protein species identification and subsequent validation using conventional methods.
Application sectors
Biomedical
Scientific Supervision:
Prof. Aldo Tomasi MD
Laboratory Head
Dott. Simone Roncioni
Staff and skills
Dott. Simone Roncioni, Laurea Magistrale in Scienze Chimiche
Dott. Francesco Burini, Laurea Magistrale in Scienze Biomolecolari e Cellulari
Dott. Luca Accorsi, Laurea Magistrale in Scienze Chimiche
Dott.ssa Elena Rebecchi, Laurea in Scienze Chimiche
Marco Palella, Tecnico Superiore per l’innovazione, sviluppo e produzione di medical devices
Instrumentation
Toxicology
High performance liquid chromatography – High resolution mass spectrometry
High performance liquid chromatography – UV spectroscopy
Gas atmospheric pressure chromatography – High resolution mass spectrometry
Climate chamber for aging tests
Proteomics
Separation system offgel
Separation system for single- and two-dimensional electrophoresis
Image acquisition and processing systems
Flow cytometer